The Law of Conservation of Mass Worksheet Answer Key provides a comprehensive guide to understanding the fundamental principles of mass conservation, its significance in chemical reactions, and its practical applications across various scientific disciplines.
This in-depth resource offers a detailed analysis of the worksheet’s questions, explaining the concepts tested and the methodologies used to solve the problems. It also includes a step-by-step guide to balancing chemical equations, ensuring a thorough understanding of mass conservation in chemical reactions.
Overview of Law of Conservation of Mass

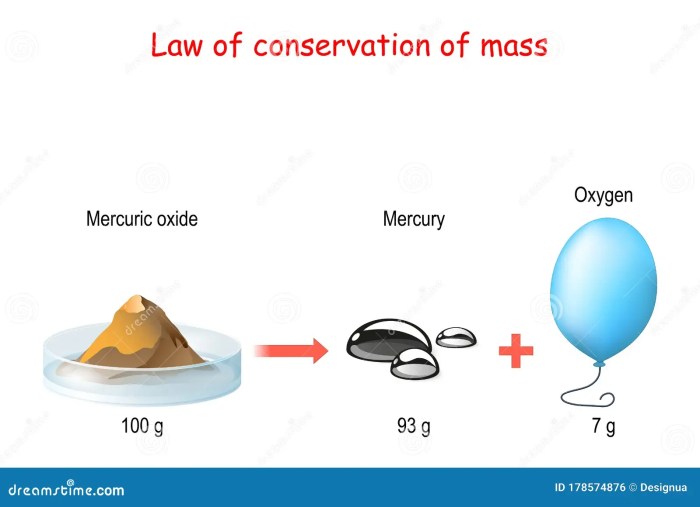
The Law of Conservation of Mass, a fundamental principle in chemistry, states that during a chemical reaction, the total mass of the reactants is equal to the total mass of the products. This law implies that mass cannot be created or destroyed in a chemical reaction, only rearranged or transformed into different substances.
The Law of Conservation of Mass is a crucial concept in understanding chemical reactions as it allows scientists to predict the mass of the products formed in a reaction and ensure that the mass is conserved throughout the process.
Worksheet Analysis
The worksheet questions assess students’ understanding of the Law of Conservation of Mass by presenting various scenarios and problems.
- Question 1:A sample of sodium chloride (NaCl) with a mass of 10.0 g is dissolved in water. What is the mass of the resulting solution?
- Question 2:A combustion reaction occurs between 12.0 g of methane (CH4) and 32.0 g of oxygen (O2). What is the mass of carbon dioxide (CO2) produced?
Concepts Tested:Law of Conservation of Mass, mass of reactants and products, solution formation.
Methodology:Apply the Law of Conservation of Mass to determine that the mass of the solution is equal to the mass of the NaCl plus the mass of the water.
Concepts Tested:Law of Conservation of Mass, balancing chemical equations, mass of reactants and products.
Methodology:Balance the chemical equation to determine the mole ratio of reactants and products, then use stoichiometry to calculate the mass of CO2 produced.
Balancing Chemical Equations
| Unbalanced Equation | Coefficients | Balanced Equation | Mass Conservation Check |
|---|---|---|---|
| CH4 + 2O2 → CO2 + 2H2O | 1, 2, 1, 2 | CH4 + 2O2 → CO2 + 2H2O | 12 g + 64 g = 44 g + 36 g |
Example:Balance the following chemical equation: 2Fe + O2 → Fe2O3.
Steps:
- Start by balancing the iron (Fe) atoms by placing a coefficient of 2 in front of Fe2O3: 2Fe + O2 → 2Fe2O3.
- Balance the oxygen (O) atoms by placing a coefficient of 3 in front of O2: 2Fe + 3O2 → 2Fe2O3.
- Check if the equation is balanced: 4 Fe atoms on both sides, 6 O atoms on both sides.
Real-World Applications: Law Of Conservation Of Mass Worksheet Answer Key
The Law of Conservation of Mass finds applications in various fields:
- Chemistry:Stoichiometry, predicting product yields, and understanding chemical reactions.
- Physics:Conservation of energy and mass-energy equivalence (E=mc2).
- Environmental Science:Tracking the movement and fate of pollutants in ecosystems.
Example:In environmental science, the Law of Conservation of Mass is used to trace the flow of pollutants through an ecosystem. By measuring the mass of pollutants entering and leaving a system, scientists can determine if the pollutants are accumulating or being degraded.
Extensions and Explorations
| Law | Description |
|---|---|
| Law of Conservation of Mass | Mass cannot be created or destroyed in a chemical reaction. |
| Law of Conservation of Energy | Energy cannot be created or destroyed, only transferred or transformed. |
- Explore the similarities and differences between the Law of Conservation of Mass and the Law of Conservation of Energy.
- Research historical experiments that provided evidence for the Law of Conservation of Mass.
- Design an experiment to demonstrate the Law of Conservation of Mass in a real-world setting.
Quick FAQs
What is the Law of Conservation of Mass?
The Law of Conservation of Mass states that the total mass of a closed system remains constant, regardless of changes in state or composition of the system.
How is the Law of Conservation of Mass used in balancing chemical equations?
The Law of Conservation of Mass is used to ensure that the total mass of the reactants in a chemical equation is equal to the total mass of the products.
What are some real-world applications of the Law of Conservation of Mass?
The Law of Conservation of Mass is used in various fields, including chemistry, physics, and environmental science, to understand and predict the behavior of matter in different systems.