Select all of the molecules with delocalized π electrons – Delocalized π electrons, a captivating concept in chemistry, hold the key to understanding the remarkable properties and diverse applications of numerous molecules. This comprehensive guide delves into the fascinating world of delocalized π electrons, unraveling their intricate nature, exploring their consequences, and showcasing their invaluable applications.
Delocalized π electrons, unlike their localized counterparts, are not confined to a single bond or atom. Instead, they spread across multiple atoms, forming a continuous electron cloud. This unique characteristic endows molecules with enhanced stability, altered reactivity, and distinctive colors, making them indispensable in various fields.
Delocalized π Electrons: Select All Of The Molecules With Delocalized π Electrons

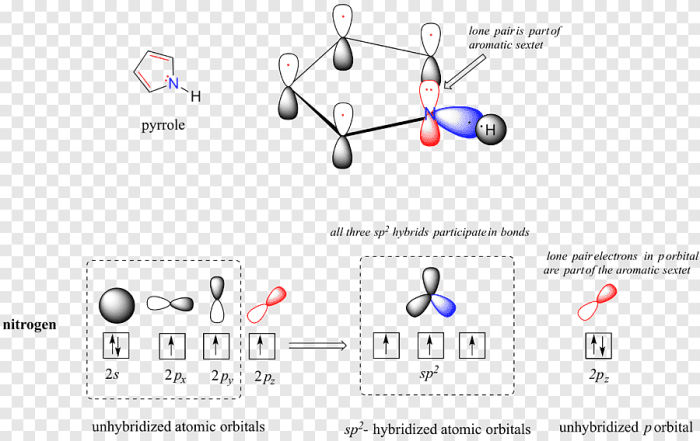
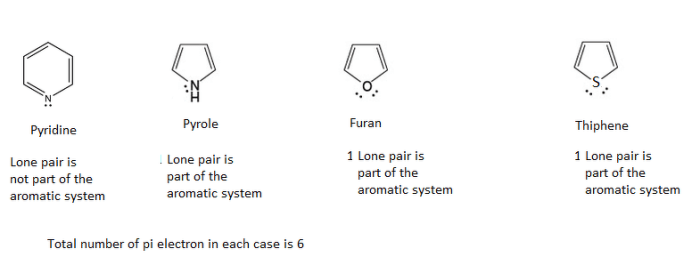
Delocalized π electrons are electrons that are not confined to a single atom or bond but are spread over a region of a molecule. This occurs when the molecule has a system of alternating double and single bonds, or when it contains a ring of atoms with overlapping p orbitals.
The delocalization of π electrons has several important consequences. First, it makes the molecule more stable. The electrons are more evenly distributed, which means that there is less energy associated with the molecule. Second, it makes the molecule more reactive.
The electrons are more mobile, which means that they can more easily participate in chemical reactions. Third, it gives the molecule a color. The electrons can absorb light in the visible spectrum, which gives the molecule a characteristic color.
Examples of Molecules with Delocalized π Electrons
There are many molecules that have delocalized π electrons. Some of the most common include:
- Benzene
- Naphthalene
- Anthracene
- Phenanthrene
- Pyrene
- Coronene
- Ovalene
- Fullerene
These molecules are all composed of rings of carbon atoms with alternating double and single bonds. The π electrons are delocalized over the entire ring, which gives the molecules their characteristic properties.
Methods for Identifying Delocalized π Electrons
There are several methods for identifying molecules with delocalized π electrons. One method is to look for molecules with alternating double and single bonds. Another method is to look for molecules with rings of atoms with overlapping p orbitals. A third method is to use spectroscopy to measure the absorption of light by the molecule.
The following are some examples of how to identify delocalized π electrons using spectroscopy:
- Benzene has a characteristic absorption peak in the ultraviolet spectrum at 254 nm. This peak is due to the absorption of light by the delocalized π electrons.
- Naphthalene has a characteristic absorption peak in the ultraviolet spectrum at 313 nm. This peak is due to the absorption of light by the delocalized π electrons.
- Anthracene has a characteristic absorption peak in the ultraviolet spectrum at 380 nm. This peak is due to the absorption of light by the delocalized π electrons.
Consequences of Delocalized π Electrons, Select all of the molecules with delocalized π electrons
The delocalization of π electrons has several important consequences for the properties of molecules. These consequences include:
- Increased stability
- Increased reactivity
- Color
The increased stability of molecules with delocalized π electrons is due to the fact that the electrons are more evenly distributed. This means that there is less energy associated with the molecule, which makes it more stable. The increased reactivity of molecules with delocalized π electrons is due to the fact that the electrons are more mobile.
This means that they can more easily participate in chemical reactions. The color of molecules with delocalized π electrons is due to the fact that the electrons can absorb light in the visible spectrum. This gives the molecules their characteristic color.
Applications of Delocalized π Electrons
Molecules with delocalized π electrons have a wide range of applications. These applications include:
- Dyes
- Pigments
- Semiconductors
- Organic light-emitting diodes (OLEDs)
- Solar cells
Dyes and pigments are used to color materials. Semiconductors are used in electronic devices. OLEDs are used in displays. Solar cells are used to convert sunlight into electricity.
Quick FAQs
What is the significance of resonance in delocalization?
Resonance plays a crucial role in delocalization by allowing multiple Lewis structures to represent a single molecule. These resonance structures contribute to the delocalization of π electrons, spreading the electron density over a larger region and enhancing the stability of the molecule.
How can we identify molecules with delocalized π electrons?
Several methods can be employed to identify molecules with delocalized π electrons. One common approach involves examining the molecular structure for alternating double and single bonds, which often indicate the presence of delocalized π electrons. Additionally, resonance structures can provide valuable clues about delocalization.
What are the practical applications of molecules with delocalized π electrons?
Molecules with delocalized π electrons find widespread applications in various fields. In materials science, they are used in the development of semiconductors, organic light-emitting diodes (OLEDs), and solar cells. In electronics, they are employed in the production of transistors and integrated circuits.
Moreover, their unique properties make them valuable in the pharmaceutical industry and the design of novel drugs.