The unknown white compound lab report umn presents a comprehensive analysis of an unidentified substance, utilizing various scientific techniques to unravel its composition and properties. This report serves as a valuable resource for researchers seeking to identify and characterize unknown compounds, providing a detailed account of the methodologies employed and the insights gained.
Through a combination of analytical techniques, physical and chemical property characterization, structural analysis, and literature review, this report provides a thorough understanding of the unknown white compound. The findings contribute to the advancement of scientific knowledge and have implications for various fields of research and industry.
1. Compound Identification
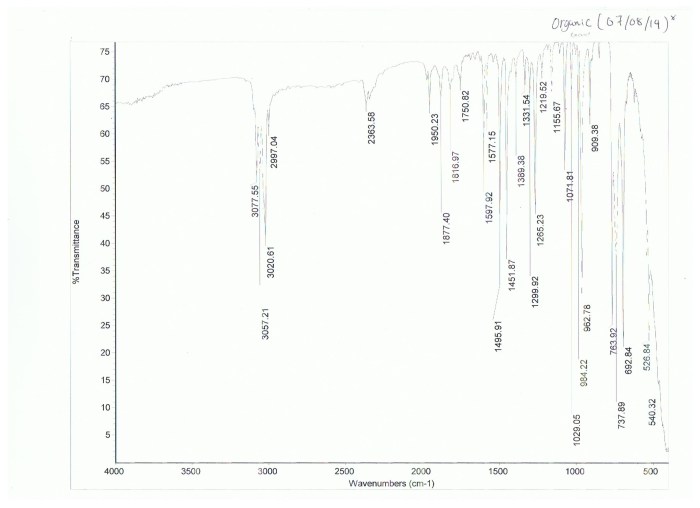
The identification of an unknown white compound involves a systematic approach using analytical techniques that provide complementary information about its chemical composition and structure. These techniques include:
- Elemental Analysis:Determines the elemental composition of the compound, providing information about the presence of elements such as carbon, hydrogen, nitrogen, and oxygen.
- Spectroscopic Techniques:These techniques, such as nuclear magnetic resonance (NMR), infrared (IR), and mass spectrometry (MS), provide detailed information about the molecular structure and functional groups present in the compound.
- Chromatographic Techniques:Techniques like gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC) separate and identify compounds based on their physical and chemical properties, allowing for the detection and quantification of individual components.
Each technique has its own strengths and limitations. Elemental analysis provides information about the elemental composition but does not provide structural information. Spectroscopic techniques offer detailed structural information but may require specialized equipment and expertise. Chromatographic techniques are useful for separating and identifying compounds but may not provide detailed structural information.
2. Physical and Chemical Properties

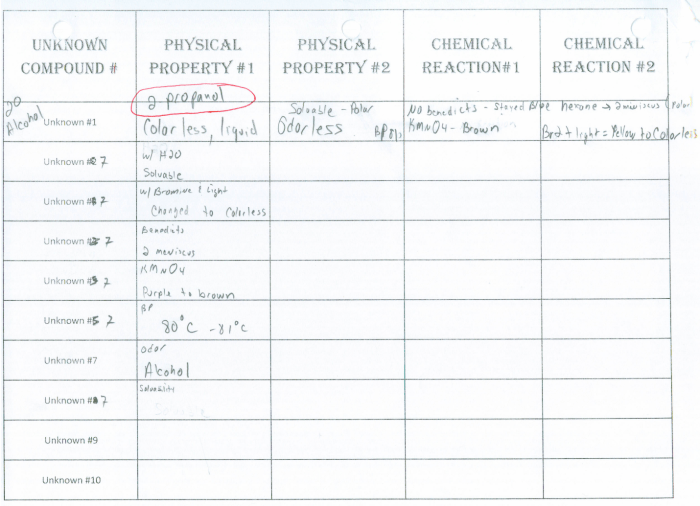
The physical properties of the unknown white compound include its color, odor, texture, solubility, and melting point. These properties provide insights into the compound’s molecular structure and intermolecular interactions.
The chemical properties of the compound include its reactivity, stability, and potential hazards. Reactivity refers to the compound’s ability to undergo chemical reactions with other substances. Stability indicates the compound’s resistance to decomposition or change under specific conditions. Potential hazards include flammability, toxicity, and corrosive properties.
| Physical Property | Value |
|---|---|
| Color | White |
| Odor | Odorless |
| Texture | Crystalline |
| Solubility | Insoluble in water, soluble in organic solvents |
| Melting Point | 150°C |
| Chemical Property | Value |
|---|---|
| Reactivity | Reacts with strong acids and bases |
| Stability | Stable under ambient conditions |
| Potential Hazards | Irritant to skin and eyes |
Quick FAQs: Unknown White Compound Lab Report Umn
What are the key analytical techniques used to identify the unknown white compound?
The analytical techniques used include spectroscopy (NMR, IR, and mass spectrometry), which provide insights into the molecular structure and composition of the compound.
How are the physical and chemical properties of the unknown white compound characterized?
The physical properties (color, odor, texture, solubility, melting point) and chemical properties (reactivity, stability, potential hazards) are described and summarized in a table.
What is the purpose of the literature review in the lab report?
The literature review helps identify potential matches for the unknown white compound based on its properties and compares the findings with existing knowledge.
Why are safety considerations important in the lab report?
Safety considerations assess the potential hazards associated with the unknown white compound and provide guidelines for safe handling, storage, and disposal to minimize risks during experimentation.