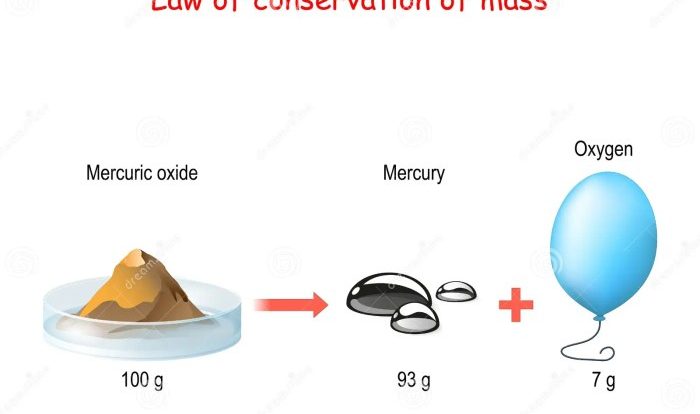
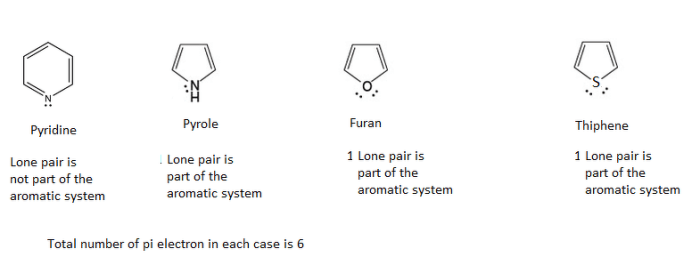
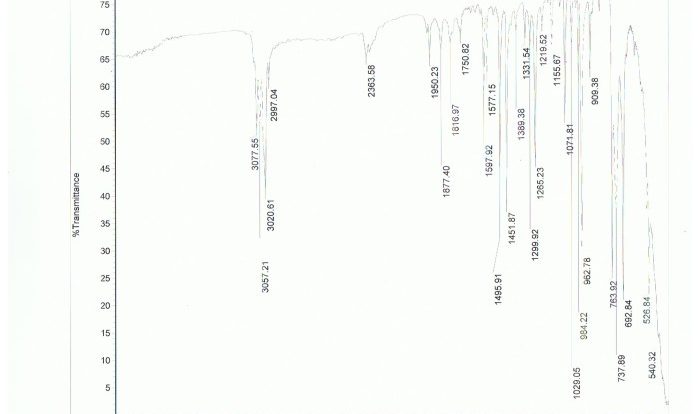
Embark on a comprehensive exploration of the Chemistry Final Exam with Answers PDF, an invaluable resource meticulously crafted to empower students with the knowledge and strategies necessary to excel in their final assessment. This guide delves into the exam’s structure, content, and potential pitfalls, providing a roadmap to success that will leave readers feeling confident and prepared.
Through an in-depth analysis of the exam’s content, students will gain a clear understanding of the key topics, concepts, and themes they need to master. The guide also identifies the various types of questions used in the exam, explaining their purpose and format, and providing ample practice opportunities with sample questions and detailed answer keys.
Chemistry Final Exam Overview
The Chemistry Final Exam is a comprehensive assessment designed to evaluate students’ understanding of the fundamental concepts, theories, and applications of chemistry covered throughout the course. The exam typically consists of multiple-choice questions, short answer questions, and essay questions. The duration of the exam is usually three hours, and the grading criteria vary depending on the specific course and instructor.The
purpose of the final exam is to assess students’ overall learning and their ability to apply their knowledge to new situations. It also helps instructors to identify areas where students may need additional support or reinforcement. The final exam grade is often a significant component of the overall course grade, and it can have a major impact on students’ academic progress.
Exam Content Analysis
The final exam encompasses a comprehensive range of topics from the chemistry curriculum, emphasizing foundational concepts, principles, and their applications.
The exam places significant weight on thermodynamics, kinetics, and equilibrium, reflecting their fundamental importance in chemical systems. Topics related to chemical bonding, structure, and reactivity are also prominently featured, highlighting the interplay between atomic and molecular properties and chemical behavior.
Thermodynamics
- Laws of thermodynamics and their implications for chemical processes
- Enthalpy, entropy, and free energy changes in reactions
- Phase transitions and their thermodynamic characteristics
Kinetics
- Reaction rates and their dependence on concentration, temperature, and activation energy
- Rate laws and mechanisms of elementary reactions
- Catalysis and its effects on reaction rates
Equilibrium
- Equilibrium constants and their applications in predicting reaction outcomes
- Le Chatelier’s principle and its use in manipulating equilibrium systems
- Solubility equilibria and their applications in precipitation and extraction
Chemical Bonding
- Types of chemical bonds (ionic, covalent, metallic)
- Molecular orbital theory and its applications in understanding bonding and reactivity
- Hybridization and its impact on molecular geometry and properties
Chemical Structure and Reactivity
- Resonance and its effects on molecular stability
- Functional groups and their characteristic reactions
- Stereochemistry and its implications for molecular properties and reactivity
Overall, the exam questions range in difficulty from straightforward applications of basic principles to more complex problems that require a deep understanding of the underlying concepts and their interrelationships.
Exam Question Types
The final exam utilizes a range of question types to comprehensively assess students’ understanding of the course material.
Each question type serves a specific purpose and is designed to evaluate different aspects of students’ knowledge and skills.
Multiple Choice Questions
Multiple choice questions present students with a set of options and require them to select the single best answer. These questions are commonly used to test students’ factual knowledge, understanding of key concepts, and ability to apply their knowledge to new situations.
Example: Which of the following is the chemical formula for water?
- H2O
- CO2
- CH4
- NH3
Short Answer Questions
Short answer questions require students to provide brief, concise answers to specific questions. These questions are used to assess students’ understanding of concepts, their ability to recall information, and their ability to communicate their knowledge effectively.
Example: Describe the process of photosynthesis.
Essay Questions
Essay questions are open-ended questions that require students to provide extended responses demonstrating their understanding of a topic. These questions are used to assess students’ ability to analyze, synthesize, and evaluate information, as well as their ability to communicate their ideas clearly and coherently.
Example: Discuss the factors that affect the rate of a chemical reaction.
Exam Preparation Strategies
Preparing for a chemistry final exam requires effective strategies to maximize understanding, recall, and problem-solving abilities. These strategies encompass reviewing course materials, practicing problem-solving, and seeking clarification from instructors.
Reviewing Course Materials
- Review lecture notes, textbooks, and other class materials thoroughly.
- Focus on understanding concepts, not just memorizing facts.
- Identify areas of weakness and spend extra time reviewing them.
Practicing Problem-Solving
- Work through practice problems and past exam questions.
- Identify common problem types and develop strategies for solving them.
- Seek help from instructors or tutors if needed.
Seeking Clarification
- Attend review sessions or office hours with instructors.
- Ask questions about concepts or problems you don’t understand.
- Discuss difficult topics with classmates or form study groups.
Exam-Taking Tips
To achieve success in the final exam, it is crucial to adopt effective test-taking strategies. These strategies encompass time management, question comprehension, and meticulous answer presentation.
Efficient time management is paramount. Allocate time wisely to each section, ensuring sufficient time for challenging questions. Prioritize questions based on difficulty and allocate more time to complex ones.
Stress Management, Chemistry final exam with answers pdf
Exam stress is common. To mitigate it, practice relaxation techniques such as deep breathing and visualization. Maintain a positive mindset, focusing on your preparation and abilities.
Time Management
- Plan your time strategically. Allocate more time to challenging questions.
- Avoid spending excessive time on any single question. Move on if you encounter difficulties and return later.
- Pace yourself throughout the exam to avoid rushing or leaving questions unanswered.
Question Comprehension
Read questions carefully and identify key concepts. Determine the type of question (e.g., multiple-choice, short answer) and answer accordingly.
Answer Presentation
Present your answers clearly and concisely. Write legibly and use proper grammar. For short answer questions, provide specific and concise responses.
Potential Exam Pitfalls
Chemistry final exams often present challenges to students, leading to common mistakes and misconceptions. Identifying these potential pitfalls is crucial for successful exam preparation and improved performance.
Understanding the exam format, time constraints, and question types is essential to avoid surprises during the exam. Students should also be aware of areas where they may struggle due to conceptual gaps or lack of practice.
Misinterpreting Questions
- Read exam questions carefully and thoroughly to grasp their intent.
- Identify s and instructions to understand what the question is asking.
- Avoid making assumptions or jumping to conclusions based on partial understanding.
Conceptual Gaps
- Review fundamental concepts and theories to ensure a strong foundation.
- Seek clarification from instructors or peers on topics that are not fully understood.
- Practice applying concepts to various scenarios to enhance comprehension.
Lack of Practice
- Solve practice problems and past exam papers to improve problem-solving skills.
- Identify areas where additional practice is needed and focus on strengthening them.
- Time yourself during practice to develop efficient time management strategies.
Time Management
- Allocate time wisely, prioritizing more challenging questions.
- Avoid spending excessive time on a single question; move on and return later if necessary.
- Use remaining time to review answers and check for any errors.
Careless Mistakes
- Proofread answers carefully to avoid errors in calculations, units, or spelling.
- Double-check numerical values, formulas, and equations to ensure accuracy.
- Write legibly to prevent misinterpretation of answers.
Sample Exam Questions
This section presents sample exam questions to help you assess your understanding of the course material. The questions cover a range of topics and question types to provide you with a comprehensive practice opportunity.
Detailed answer keys or explanations are provided for each question to facilitate your learning and ensure you understand the correct reasoning and concepts.
Chemical Bonding
- Define chemical bonding and explain the different types of chemical bonds.
- Draw the Lewis structure for carbon dioxide (CO 2) and predict its molecular geometry.
- Explain the concept of hybridization and its role in determining molecular shape.
Thermochemistry
- Calculate the enthalpy change (ΔH) for a reaction using Hess’s law.
- Describe the different types of thermochemical reactions and their applications.
- Explain the concept of entropy and its relationship to spontaneity.
Chemical Kinetics
- Define the rate of a reaction and explain the factors that affect it.
- Write the rate law for a given reaction and determine the order of the reaction with respect to each reactant.
- Explain the concept of activation energy and its role in reaction rates.
Equilibrium
- Define chemical equilibrium and explain the Le Chatelier’s principle.
- Calculate the equilibrium constant (K) for a reaction and explain its significance.
- Explain the factors that affect the position of equilibrium.
Acids and Bases
- Define acids and bases according to the Brønsted-Lowry theory.
- Calculate the pH of a solution and explain the concept of buffers.
- Explain the relationship between acid strength and base strength.
Electrochemistry
- Define oxidation and reduction and explain the role of half-reactions.
- Calculate the standard cell potential (E°) for a given electrochemical cell.
- Explain the factors that affect the spontaneity of electrochemical reactions.
Organic Chemistry
- Identify and name functional groups in organic compounds.
- Draw the structural formula for a given organic compound based on its IUPAC name.
- Explain the concept of resonance and its implications for organic compound stability.
Additional Resources: Chemistry Final Exam With Answers Pdf
Students are encouraged to utilize a range of resources to enhance their preparation for the final exam. These resources can provide additional practice, reinforce concepts, and offer alternative perspectives on the course material.
The following resources are recommended:
Online Study Materials
- Khan Academy: Offers free online video lessons, practice exercises, and assessments covering a wide range of chemistry topics. (https://www.khanacademy.org/science/chemistry)
- Crash Course Chemistry: Provides engaging video lessons that cover key chemistry concepts in a concise and accessible format. (https://www.youtube.com/playlist?list=PL8dPuuaLjXtOfse2ncvffeelTrqvhrz8H)
- Chemistry LibreTexts: Offers a comprehensive online textbook that covers the entire scope of general chemistry. (https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_ChemPRIME_(Moore_et_al.))
Practice Tests
- Previous Exam Papers: Obtain past exam papers from your instructor or the course website. These provide valuable insights into the exam format, question types, and difficulty level.
- Online Practice Tests: Utilize online platforms such as (https://www.exampro.com/practice-tests/chemistry/) and (https://www.kaptest.com/study/chemistry-practice-test) to take practice tests and receive immediate feedback.
- Textbook Practice Problems: Work through the practice problems provided at the end of each chapter in your textbook. These problems reinforce the concepts covered in the chapter and provide an opportunity to apply your knowledge.
Relevant Textbooks and Websites
- Textbook: Refer to your assigned textbook for in-depth explanations, worked examples, and additional practice problems.
- Royal Society of Chemistry: Access a wealth of resources including articles, videos, and interactive simulations. (https://www.rsc.org/education/)
- American Chemical Society: Explore a variety of resources such as journal articles, career information, and educational materials. (https://www.acs.org/)
Helpful Answers
What is the purpose of the Chemistry Final Exam?
The Chemistry Final Exam is a comprehensive assessment designed to evaluate students’ understanding of the key concepts, principles, and theories covered throughout the chemistry course.
How can I effectively prepare for the Chemistry Final Exam?
Effective preparation involves reviewing course materials, practicing problem-solving, seeking clarification from instructors, and utilizing additional resources such as study guides and practice tests.
What types of questions can I expect on the Chemistry Final Exam?
The Chemistry Final Exam typically includes a variety of question types, such as multiple choice, short answer, and essay questions, each designed to assess different levels of understanding and critical thinking.
How can I manage my time effectively during the Chemistry Final Exam?
Effective time management involves allocating time wisely to each question, prioritizing difficult questions, and utilizing strategies such as the Pomodoro Technique to maintain focus and productivity.
What resources are available to help me prepare for the Chemistry Final Exam?
Numerous resources are available, including online study materials, practice tests, textbooks, and websites, which provide additional practice opportunities and reinforce concepts covered in class.